Reproductive Behavior, Embryology, and Larval Development of Four Species of Pygmy Sunfish
By Maurice F. Mettee and Christopher Scharpfreprinted from American Currents, Winter (Feb.) 1998
Introduction: Are Pygmy Sunfishes Sunfishes?
The pygmy sunfishes of the genus Elassoma include six described species, all of
which are native to swamps and backwater areas of the southeastern United States and
middle Mississippi Basin:
• Elassoma zonatum Jordan 1877, the
banded pygmy sunfish, is the most widespread,
found from North Carolina through the
southeastern states into western Texas and
Oklahoma and northward into southern Illinois.
A zonatum-type specimen collected in
a Rotenone survey in Tennessee may represent a
new, undescribed species.
• Elassoma evergladei Jordan
1884, the Everglades pygmy sunfish, extends from South
Carolina into Florida and westward to the
Mobile drainage in southern Alabama.
• Elassoma okefenokee Böhlke
1956, the Okefenokee pygmy sunfish, is restricted to
southern Georgia and the northern third of
Florida. A population in northwest Florida
appears to be a distinct, undescribed species.
• Elassoma boehlkei Rohde and
Arndt 1987, the Carolina pygmy sunfish, is found in
the Waccamaw and Santee River drainages of
North and South Carolina.
• Elassoma okatie Rohde and
Arndt 1987, the bluebarred pygmy sunfish, is endemic
to three river drainages in South Carolina:
Lower Edisto, New and Savannah.
• Elassoma alabamae Mayden 1993,
the spring pygmy sunfish, was previously known
from collections at only two locations in
Alabama: Cave Spring, Lauderdale County, in
1937; and Pryor Spring, Limestone County, in
1941. No other specimens were seen for
more than 30 years, leading most ichthyologists
to conclude the species was extinct. But
in 1973, David A. Etnier discovered a new
population at Moss Spring, a tributary of
Beaverdam Creek in Limestone County. Since then
additional populations have been found
within the Beaverdam Creek system. In 1984, the
senior author was part of a team of local
landowners and state and federal biologists who
worked together to successfully introduce
gravid adults from Beaverdam Creek into Pryor
Spring (Mettee and Pulliam, 1986).
Since the description of E. zonatum in 1877, the taxonomic position of the genus Elassoma has been the subject of much controversy. Originally, the fish was thought to be a cichlid (Jordan, 1877), but that opinion soon changed. Hay (1881) and Jordan and Gilbert (1882) placed E. zonatum into its own family, the Elassomatidae, because they believed it was intermediate between the pirate perch (family Aphredoderidae) and the centrarchids. Boulenger (1895) placed Elassoma into the sunfish family Centrarchidae because of the similarity and kinds of vertebrae; he was also probably the first to postulate that Elassoma was a dwarf sunfish.
Over the years, several investigators have presented evidence they felt was sufficient to exclude Elassoma from the Centrarchidae. After examining the olfactory organs of three centrarchid species and E. zonatum, Eaton (1956) stated that Elassoma was a neotenous sunfish—that is, capable of being sexually mature as a juvenile. Branson and Moore (1962) surveyed the acoustico-lateralis systems of 26 centrarchid species and the three described Elassoma species at that time; they concluded that while elassomids were closely related to centrarchids and possibly shared a common ancestry with them, they had specialized and diverged sufficiently to be considered a separate family. Moore and Sisk (1963) stated that the eye structure of Lepomis and Elassoma were markedly different. Roberts (1964) examined the chromosome complements of 20 centrarchid species and found that while E. zonatum possessed the modal centrarchid number of 48 diploid chromosomes, its chromosome morphology differed significantly from that of any other centrarchid species. This led him to the conclusion that while Elassoma is distantly related to the sunfishes, it still differs to the extent that it should be placed in a separate family. Similar findings based on biochemical studies were presented by Avise and Smith (1977).
One aspect of the life history of Elassoma which has received little investigation, but which might be important in providing additional information regarding its relationships to centrarchids, is reproductive behavior. Because elassomid fishes are usually found in slow-moving waters that are less than one foot deep and choked with aquatic vegetation, observations on their reproductive behavior would be difficult; consequently, all reports on their spawning behavior have been based on aquarium experiments.
Several investigators have indicated that the spawning habits of elassomid fishes are similar to those of other centrarchids, since the male constructs a nest into which the eggs are deposited during spawning. Such behavior was observed in E. evergladei by Axelrod and Shaw (1971), Innes (1969), and Axelrod and Schultz (1971). Contrarily, Nachstedt and Tusche (1954), Sterba (1961), Breder and Rosen (1966), and Branson (1974) stated that this species was not a nest builder. Shortt (1956) observed that the eggs of E. okefenokee were deposited in "moss," but did mention a nest. After he had observed spawns of E. zonatum in aquaria, Poyser (1919) speculated that this species preferred to spawn over a nest, but if bottom conditions were unfavorable, it would alternately spawn on algae or aquatic vegetation. In a paper on the life history and ecology of E. zonatum at Mound, Louisiana, Barney and Anson (1920) noted that the eggs of this species were always found scattered about in the aquatic vegetation. It is obvious from this summary that the reproductive behavior of Elassoma is incompletely known and in need of additional research before it can be compared to that of the Centrarchidae.
This article is an adaptation of the senior author’s Ph.D. dissertation (Mettee,
1974), which documented the reproductive behavior of the four Elassoma species
known at the time—zonatum, okefenokee, evergladei, and the then undescribed alabamae—with
the intent of comparing it with that of the family Centrarchidae. Composite descriptions
of the embryology of elassomid fishes were also presented, as well as information on
growth rates and fin development.
Materials and Methods,
with Notes on Aquarium Care
All reproductive studies were conducted in the laboratory. The fishes were contained in four 40-liter and six 20-liter all-glass aquaria. Continuous air was supplied by aquarium pumps and air stones. Water temperature was controlled within 3°C by tube-type aquarium heaters with internal thermostats. A 15.5 hour light period was maintained throughout the study using daylight supplemented with fluorescent light banks on an automatic timer. Because elassomid fishes will not readily eat dry foods, they were fed live brine shrimp (Artemia) nauplii either daily or every second day, depending upon their size and breeding condition.
In order to duplicate their natural environment as closely as possible, aquatic plants, principally of the genus Ceratophyllum, were collected with breeding stocks of elassomid fishes and used in the spawning aquaria. Specimens were transported from the field to the lab in Styrofoam boxes and then placed into a 40-liter aquarium filled with 21°C distilled water. After a period of 7-10 days, five or six mature females were transferred into each of two 20-liter aquaria with physical conditions similar to those of the holding tank. Using aquarium heaters, the water temperature in these two aquaria was gradually raised 2.5-4.5°C over a period of 10-14 days until the female abdomens began to enlarge, indicating egg production. This temperature was maintained for another 7-8 days, at which time one or two males of the same species were introduced into each tank with the females. After a period of 2-3 days, during which the males established territories, spawning usually occurred.
Within 10 minutes after spawning, the eggs were transferred into 50 ml petri dishes and maintained under similar physical conditions. Photographs of live eggs were taken of each embryological stage, upon which the accompanying composite illustrations were drawn.
The prolarvae were maintained in the same petri dishes until they reached
a total length of 8-10 mm. At this time they were transferred into a 20-liter all-glass
aquarium and allowed to grow to adult size. Periodically, specimens were preserved in a 5%
formalin solution for later observation. Because of the small number of eggs produced by a
single spawn, and the high mortality rates of eggs and larvae, several spawns were
necessary in order to complete a series from newly hatched prolarvae to adult.
Behavior
Based on the following observations, the reproductive behavior of the four Elassoma species studied is very similar.
Breeding Coloration
Prior to and during the spawning period, the males of each Elassoma species became
very brightly colored, while the females retained their characteristic olive-to-tan color
with dark brown mottling, scattered dots and/or irregular bars. Except for the bright
blue, symmetrical band in the dorsal and anal fins of the latter, the color pattern of
breeding males of E. evergladei and E. okefenokee was very similar. During
periods of active spawning, males of both species assumed a velvety black color. Located
posteriorly to the head on each were 7-9 irregularly spaced, vertical, iridescent
turquoise (E. evergladei) or blue (E. okefenokee) bars, 1-2 mm wide,
extending the full depth of the body. A small vertical, iridescent blue bar, approximately
3 mm long, developed immediately posterior to each eye and joined another bar similar in
length and color that extended horizontally below the eye. The dorsal and anal fin
membranes of both species were dusky to black with one or two rows of small translucent
dots that were more noticeable in the posterior half of each fin and became obliterated
anteriorly. The pelvic and caudal fins were dusky and without dots; however, the distal
ends of the pelvic fin membranes of E. okefenokee were tipped in bright blue. The
pectoral fins of both species remained clear to slightly dusky.
The color pattern of breeding males of E. zonatum was essentially unchanged, although the colors did intensify considerably. All of the fin membranes, except those in the pectoral fins, became much darker, and the 9-11 vertical bars on the trunk darkened to the extent that the black spot usually found ventral to the dorsal fin origin was indistinguishable. A small crescent similar in size and position to the ones described for E. evergladei and E. okefenokee, but gold in color, was present around the eye, and many small, iridescent gold and blue flecks were scattered about on the cheeks and opercula.
Breeding males of E. alabamae were dark brown to black, and on the trunk were located 6-8 very narrow, irregularly spaced, vertical, iridescent gold bars that extended the entire body depth. An iridescent gold structure similar to that described for E. zonatum was present around each eye, perhaps the most outstanding color characteristic of males of this species was a distinct, clear spot in each of the last four dorsal fin and anal fin membranes which, when viewed collectively, formed a "window" in the posterior end of each fin. This "window" is a valuable key character for this species as it was present in the dorsal and anal fins of all male individuals used in this study.
The Sidling Threat Display
When one or two males were placed into an aquarium with several females of the same
species, each immediately selected a territory that was approximately 125 x 125 mm,
extending from the surface of the water almost to the bottom in one corner. Females
usually remained at or near the bottom. Gravid females could travel through a male’s
territory unmolested, but if another male or non-gravid female approached, a confrontation
called the Sidling Threat Display by Miller (1964) occurred. During this display, the male
whose territory had been violated swam to within 30-50 mm of the intruder and expanded his
fins almost to their fullest extent. The caudal and pectoral fins "beat" very
rapidly and the male’s color intensified, indicating his apparent "anger"
at his opponent. The male next turned himself broadside or nearly so in order to present
the image of a larger fish and, thereby, possibly scare the intruder into retreat. If this
failed, the male, while moving closer to his adversary, would arc his body so that his
head and tail were closer to the intruder; when within range he would strike at him very
quickly. The strike was accomplished with such haste that it was impossible to tell if
physical contact had actually occurred. No physical damage to either fish was ever
observed after these skirmishes. As a result of this display, the intruder usually
retreated hastily from the territory and occasionally the victorious male would chase him
to the opposite end of the aquarium.
The Wiggle Waggle Display
If a potential spawning partner entered a male’s territory, another behavior pattern
called the Wiggle Waggle Display by Miller (1964), was observed. The male would approach
the female very slowly, and if she did not swim away, he would begin an erratic dance
which consisted of swimming toward the potential spawning area in an up-and-down pattern,
raising and lowering his dorsal and anal fins, and extending and flexing his pelvic fins
alternately (Figure 1). These gestures were repeated several times by the male, always in
the direction of the aquatic vegetation that he had previously selected as the potential
spawning site. In his apparent "impatience" to spawn, the male would bite the
female, which usually sent her in a hasty retreat. But if he was persistent, the male
would eventually persuade the female to accompany him to the spawning area. Once the
female entered the aquatic vegetation, the male became brightly colored, and his body
began to quiver as he gently nipped the female’s genital papilla and nudged her
abdomen on one side and then the other. During this time the female would position herself
in the aquatic vegetation once or twice, presumably to select the best position for egg
deposition. The male continued his activities for 2-3 minutes after which he aligned
himself on one side of the female. While both fishes remained in the upright position, the
eggs and sperm were extruded.
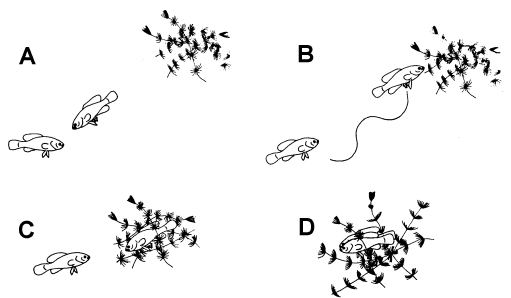
Figure 1. Stages of the reproductive behavior of elassomid fishes. A = male approaching female. B = wiggle waggle dance of male. C = female approaching the spawning site. D = the spawning act.
Vegetation and Egg Deposition
Most of the eggs fell into the fine-leafed Ceratophyllum, where they would stick in small
clusters; however, it was common for one or two eggs to drop through to the bottom of the
aquarium. After both participants rested briefly, the male chased the female from the
spawning site, as she would cannibalize her own eggs. The entire spawning act lasted from
5-6 minutes.
Depending on the species, the male continued to guard the eggs for the next 72-100 hours. If another individual approached, it was confronted by a Sidling Threat Display and chased from the area. When the eggs were being collected for observation, it was not uncommon for the male to bite on the end of the pipette; if that failed to stall collection efforts, he would eat his own eggs. Once the eggs were removed from the spawning site, the male would renew his efforts to spawn with another female.
After witnessing several spawns of each of the four elassomid species, it became evident that the lack of aquatic vegetation as a suitable spawning medium may have been the reason why Axelrod and Shaw (1967), Innes (1969) and Axelrod and Schultz (1971) have observed these fishes spawning on the bottom rather than in aquatic vegetation. As mentioned in the Materials and Methods section, most of the vegetation collected with elassomid breeding stock was Ceratophyllum, a thick-growing, fine-leafed plant. Elassomid eggs were always found attached to leaves of Ceratophyllum, except in cases where this plant was either not available or in a decomposing state, at which time the eggs were found on the bottom. Because of their semi-adhesive nature, the eggs would become covered with debris soon after they reached the bottom of the aquarium. In his efforts to clear away the debris, the male would clean an area that could be construed as a nest by those familiar with the bedding habits of centrarchid species. Photographs that lend support to this idea were given in Axelrod and Shaw (1967). The first sequence of photographs showed specimens of E. evergladei spawning in what appeared to be dying strands of Ceratophyllum or some closely related plant on the bottom of the aquarium, while the second photograph depicted a male E. evergladei guarding eggs that had been scattered about in healthy strands of aquatic vegetation that were floating away from the bottom.
Comparison and Conclusion
The following aspects of the reproductive behavior of elassomid fishes contrast with the
behavior patterns of centrarchid species as outlined in Breder and Rosen (1966):
1. Unlike centrarchids, elassomids did not construct nests for egg deposition.
2. When given the proper spawning medium, elassomids always spawned in aquatic vegetation
above the bottom.
3. The displays of male elassomids described herein and by Miller (1964) are more complex
than those previously reported for any centrarchid species.
4. Both male and female elassomid fishes remained in the upright position during the
spawning act, while in most centrarchid species the female assumed an inclined position
when the eggs were released.
A later study by Walsh and Burr (1984) confirms that E. zonatum, like other pygmy sunfishes, deposits its eggs in aquatic vegetation rather than in cleared nests.
According to Mayr (1969), behavioral taxonomic characters are often superior to
morphological characters in the study of two closely related groups. From this study, it
is evident that the behavior of elassomid and centrarchid fishes is not similar. Based on
the morphological, chromosomal, biochemical and behavioral differences, as given in Eaton
(1956), Branson and Moore (1962), Roberts (1964), Avise and Smith (1977), and this study,
it is our opinion that the elassomid fishes have specialized to an extent to justify their
being placed into a separate family, the Elassomatidae.
Embryology
The embryological stages illustrated in Figure 2 are based on the observation of eggs
collected from 17 spawns of E. okefenokee, 10 spawns of E. evergladei, six
spawns of E. alabamae, and 24 spawns of E. zonatum. Because there is
variability in developmental rates within groups of eggs from the same spawn, observations
were made on a time schedule, and developmental stages were assigned based on the stage
demonstrated by the majority of eggs at that time.
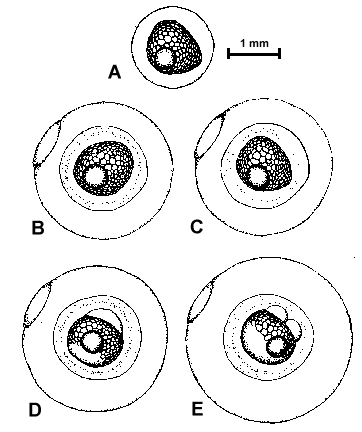
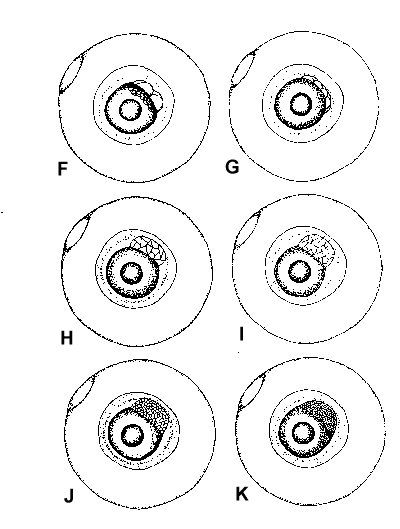
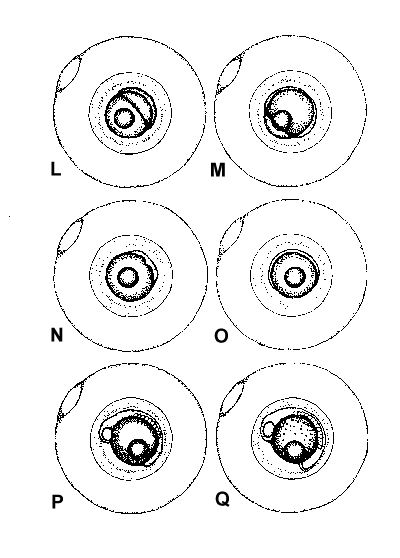
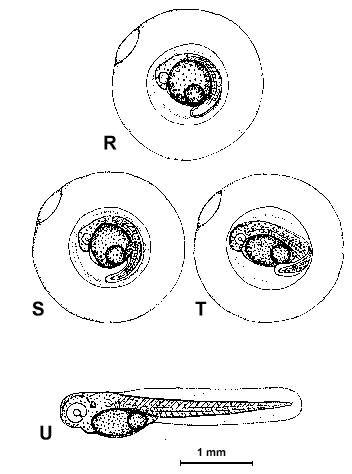
Figure 2. Composite illustrations of the embryology of elassomid fishes. A = unfertilized egg. B, C = fertilized egg within 10 minutes after fertilization. D = one-celled embryo. E = two-celled embryo. F = four-celled embryo. G = eight-celled embryo. H = 16-celled embryo. I = 32- to 64-celled embryo. J = early high blastula. K = late high blastula. L = early gastrula. M = late gastrula. N = neurula, end view. O = neurula, lateral view. P = early larval stage. Q = 12-14 somite stage. R = 16-18 somite stage. S = 20-24 somite stage; brain and eyes prominent; heart is pumping colorless blood. T = prehatch larva; morphological development of larva appeared complete; all areas of brain visible; blood cells light pink in color. U = newly hatched larva.
The time period from fertilization to hatching is given in Table 1. A comparison of
fecundity rates (number of eggs fertilized) and percent survival of eggs is found in Table
2. Egg diameters immediately after spawning and at hatching time are given in Table 3.
Table 1. Developmental rates for four species of Elassoma.
| Developmental Stages |
Species, Incubation Temperatures, and Times |
|||||||
| E. okefenokee (23°C) |
E. evergladei (25.5°C) |
E. alabamae (23.5°C) |
E. zonatum (21°C) |
|||||
| Hours | Minutes | Hours | Minutes | Hours | Minutes | Hours | Minutes | |
| One-celled embryo | 30 | 20 | 30 | 45 | ||||
| Two-celled embryo | 50 | 40 | 50 | 1 | 20 | |||
| Four-celled embryo | 1 | 30 | 1 | 1 | 30 | 2 | ||
| Eight-celled embryo | 2 | 1 | 45 | 2 | 10 | 2 | 55 | |
| 16-celled embryo | 2 | 45 | 2 | 30 | 3 | 4 | ||
| 32- to 64-celled embryo | 4 | 3 | 15 | 4 | 30 | 7 | ||
| Early high blastula | 7 | 4 | 8 | 9 | 15 | |||
| Late high blastula | 10 | 8 | 9 | 15 | 11 | 30 | ||
| Early gastrula | 12 | 10 | 12 | 13 | ||||
| Late gastrula | 15 | 14 | 13 | 16 | ||||
| Neurula | 17 | 15 | 14 | 19 | ||||
| Early larval stage | 27 | 25 | 22 | 29 | ||||
| 12-24 somite stage | 30 | 28 | 27 | 35 | ||||
| 16-18 somite stage | 38 | 35 | 32 | 43 | ||||
| 20-24 somite stage | 50 | 45 | 40 | 53 | ||||
| Prehatch larva | 70 | 60 | 52 | 72 | ||||
| Beginning to hatch | 82 | 65 | 72 | 110 | ||||
Table 2. Comparison of fecundity range and percent survival of eggs for the four
species of Elassoma.
Species |
Fecundity Range |
Percent Egg Survival |
E. okefenokee |
20 to 25 |
45 to 50 |
E. evergladei |
25 to 30 |
50 to 55 |
E. alabamae |
60 to 65 |
35 to 40 |
E. zonatum |
20 to 68 |
55 to 60 |
Table 3. Egg diameters of four species of Elassoma immediately after
spawning and at hatching time.
| Species |
Egg Diameters in Millimeters |
|
After Spawn |
At Hatching Time |
|
E. okefenokee |
1.5 to 1.7 |
2.0 to 2.1 |
E. evergladei |
1.4 to 1.2 |
2.1 to 2.2 |
E. alabamae |
2.2 to 2.3 |
3.0 to 3.2 |
E. zonatum |
2.6 to 2.7 |
3.7 to 3.8 |
During the hatching process, movements inside the egg became more frequent
and violent until eventually, by using the tail as a lever, the larva ruptured the
chorion, freeing the posterior end of its body. After a short rest the larva would shake
itself free.
Description of the Prolarvae and Postlarvae
Prior to metamorphosis, elassomid larvae cannot be distinguished from each other; therefore, descriptions included herein pertain to pygmy sunfish larvae in general unless otherwise specified.
Newly hatched larvae (Figure 2-U) were tadpole-like in shape, except for a large ventro-lateral bulge caused by the enlarged yolk sak. No mouth was visible. The eyes were without pigment. A small pectoral fin bud which consisted of a fan-shaped membrane without fin ray primordia was present on either side of the larvae posterior to the eye and dorsal to the yolk sac. No pelvic fin buds were present. The major areas of the brain were distinguishable. The heart beat rate remained at 100-115 beats per minute for E. okefenokee, 145-150 for E. evergladei, 140-144 for E. alabamae and 134-136 for E. zonatum, and the blood pathway around the yolk sac and through the vessels of the body was visible. When viewed from the dorsal side, four pairs of gill arches and the rhythmic movements of the gill covers were observed.
Several morphological and behavioral changes were observed after the transition from
prolarval to postlarval stages. The standard lengths at which these changes occurred are
given for each species in Table 4.
Table 4. Standard lengths (mm) at which Elassoma change from prolarvae to postlarvae and postlarvae to juveniles.
| Species | Prolarvae to Postlarvae | Postlarvae to Juvenile |
| E. okefenokee | 3.2 to 3.4 | 8.0 to 9.0 |
| E. evergladei | 3.4 to 3.5 | 6.4 to 7.0 |
| E. alabamae | 3.4 to 3.5 | 5.3 to 5.7 |
| E. zonatum | 3.5 to 3.7 | 8.0 to 8.5 |
Because of its cumbersome yolk sac and lack of functional fins, the prolarvae spent most
of their time lying on their sides on the bottom. Periodically, they would swim in short
rapid spurts. Eyesight was apparently very poor since the larvae would often collide with
each other or frequently swim headlong into the side of the petri dish. As the yolk sac
was absorbed, the larvae, by "beating" their pectoral fins buds very rapidly,
would balance themselves in an upright position for short periods of time. This behavior
became more frequent until by the fifth day after hatching; they remained in the upright
position most of the time. In the first two or three days after hatching, eye color of the
larvae began to darken; by day four or five it was completely black. Eyesight and other
sensory perception were apparently much improved at this time; when brine shrimp nauplii
were introduced into the petri dishes, the larvae had no difficulty in catching and eating
them. Species color development did not begin until after metamorphosis and at standard
lengths of over 9.0 mm.
Food and Larval Mortality
As stated in Lagler, Bardach and Miller (1962), food is a primary concern to the
prolarvae. Due to the lack of a suitable food source, the greatest mortalities occur
during the first few days after hatching. Mortality rates for elassomid larvae varied
between 45-55% for the first week after hatching. Using the larvae of four marine species,
Farris (1959) demonstrated that prolarvae undergo three distinct growth periods after
hatching. Initially, there was a period of rapid growth followed by a period of slower
growth. The third and most critical stage followed the absorption of the yolk materials,
when the larvae had to metabolize themselves until they could actively feed. This was when
the largest mortalities occurred. Fortunately, a number of the larvae of each elassomid
species lived through the "critical phase" between yolk absorption and active
feeding, but those that survived grew smaller. Larval shrinkage was observed on day 4, and
recovery occurred on days 7-10.
Temperature and Larval Growth
The time period necessary for newly hatched prolarvae to grow to
adult size (approximately 16 mm standard length) was 160 days for E. okefenokee, 90
days for E. evergladei, 325 days for E. alabamae, and 100 days for E.
zonatum.
In his discussion on larval metabolism and growth, Blaxter (1969) indicated that
ambient temperature was one of the most important influences on the rate of development.
During this study, individuals of E. okefenokee and E. zonatum that were
maintained at lower temperatures (23°C and 21°C, respectively) metamorphised at an older
and longer standard length than did specimens of E. evergladei. The growth pattern
for individuals of E. alabamae differed from the other elassomid species. Even
though they were maintained at a lower temperature (23.5°C), the larva of this species
grew faster than those of E. evergladei (25.5°C). Once they had lived through the
"critical phase" between the time of yolk absorption and active feeding, growth
in E. alabamae larvae was rapid for approximately 40 days, after which it slowed
significantly for 270 days.
Fin Development
Pen drawings of the sequence of fin development in elassomid fishes are given in Figure 3. The standard lengths at which various fin primordia were first observed are listed in Table 5.
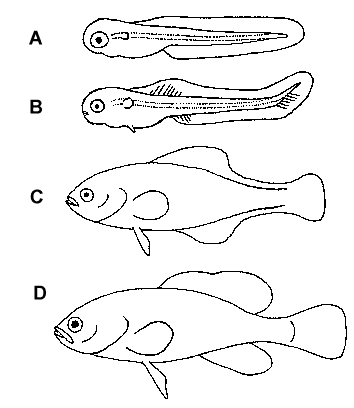
Figure 3. Stages of fin development. A = newly hatched prolarva. B =
postlarva with fin fold primordia. C = late postlarva with fin fold remnants. D = young
adult.
Table 5. Standard lengths (mm) at which fin primordia were first observed
in four species of Elassoma.
| Species | Caudal Fin | Dorsal and Anal Fin | Pelvic Fins |
| E. okefenokee | 4.9 | 5.9 | 5.5 |
| E. evergladei | 4.4 | 4.6 | 5.5 |
| E. alabamae | 4.3 | 5.0 | 5.3 |
| E. zonatum | 4.9 | 5.7 | 5.5 |
All larvae hatched with a pair of pectoral fin buds and a continuous fin fold, neither of
which contained fin rays. No pelvic fins were present. At approximately 3.8-5.0 mm,
depending on the species, the posterior end of the notochord and associated fin fold
turned dorsally, temporarily forming a heterocercal tail. Shortly thereafter, caudal fin
primordia were observed developing out from the posterior edge of the notochord. Several
caudal fin primordia were present before the first dorsal and anal fin primordia were
seen. The fin fold remained intact until most of the dorsal and anal fin primordia were
present, at which time it began to decrease in depth until the sections joining the dorsal
and anal fins to the caudal fin disappeared. Ray formation proceeded from the ventral to
the dorsal margins in the pectoral and caudal fins, and posterior to anterior in the
dorsal and anal fins. By the time that the anterior spines were developing in the dorsal
and anal fins, the soft rays of those fins were beginning to branch. Although it was
extremely difficult to observe them because of their small size, soft rays appeared to
develop before the single spine in each pelvic fin.
Fin development was complete at standard lengths of 8.0-9.0 mm for E. okefenokee, 6.4-7.0 for E. evergladei, 5.3-5.7 for E. alabamae, and 8.0-8.5 for E. zonatum.
Squamation
The fishes of all four elassomid species studied here are covered with cycloid scales except for E. okefenokee and E. alabamae, which do not have scales on the tops of their heads. Small scales were first observed on late prolarvae of each species; by the time metamorphosis had occurred, scales covered the entire body.
Epilogue: The Changing Face of Elassomid Systematics
Since the completion of the senior author’s initial study on elassomids, several additional studies have come forth indicating an even more distant relationship between pygmy sunfishes and centrarchids, and perhaps no relationship at all. Humphries and Lauder (1985) found no evidence to support the notion that elassomids are a sister group of centrarchids. Johnson (1984, 1993) presented evidence that elassomid affinities lie outside the Percoidei, the large perciform suborder that includes such familiar fishes as groupers, perches and darters, butterflyfishes, marine angelfishes, and sunfishes. Johnson and Patterson (1993) expounded on this belief, finding that elassomids share some derived features with synbranchids (swamp eels), mugilomorphs (mullets), gasterosteiformes (sticklebacks, seahorses, etc.), mastacembelids (spiny eels), and atherinomorphs (rainbowfishes, killifishes, etc.). They even proposed a name for this new group—Smegmamorpha—an acronym using the initials (S-M-E-G-M-A) of the six taxa which comprise the group. In addition, the name derives from the Greek and Latin smegma, meaning cleansing or cleansing agent. In this usage, the name refers to the authors’ "expectation that grouping these taxa will have the effect of cleaning up or tidying the systematics of higher teleosts . . .".
More recently, Johnson and Springer (1997) presented evidence that in every aspect an elassomid’s skeleton is trying to be like a stickleback’s. A formal rationale for placing elassomids into Gasterosteiformes is being prepared (G. D. Johnson, pers. comm. with CS).
Until their relationships are more clearly and definitively resolved, most
ichthyologists retain pygmy sunfishes in the order Perciformes, within their own suborder
(Elassomoidea) and family (Elassomatidae) (Nelson, 1994; Helfman et al., 1997). Please
note, however, that many publications, including the popular How to Know the Freshwater
Fishes (Eddy and Underhill, 1978) and the American Fisheries Society list of common and
scientific names (Robins et al., 1991), still place pygmy sunfishes among the
centrarchids. This will no doubt change in future editions.
Literature Cited
Avise, J. C. and M. H. Smith. 1977. Gene frequency comparisons between sunfish
(Centrarchidae) populations at various stages of evolutionary divergence. Syst. Zool.
26:319-335.
Axelrod, H. R. and L. P. Schultz. 1971. Handbook of tropical aquarium fishes. T.F.H. Publications, Inc., Jersey City. 718 p.
---------- and S. R. Shaw. 1967. Breeding aquarium fishes. Book I. T.F.H. Publications, Inc., Hong Kong. 480 p.
Barney, R. L. and B. J. Anson. 1920. Life history and ecology of pygmy sunfish, Elassoma zonatum. Ecology. 1:241-256.
Blaxter, J. H. S. 1969. Development: eggs and larvae, p. 178-252. In W. S. Hoar and D. J. Randall (Eds.). Fish Physiology. Vol. 3. Academic Press, New York.
Böhlke, J. E. 1956. A new pygmy sunfish from southern Georgia. Notulae Naturae. 294. 11 p.
Boulenger, G. A. 1895. A catalogue of fishes of the British Museum. 2nd ed. Order of Trustees, London. Vol. 1. 349 p.
Branson, B. A. 1974. Pygmy sunfish for community aquaria. Tropical Fish Hobbyist. XXII. 17-22.
---------- and G. A. Moore. 1962. The lateralis components of the acoustico-lateralis system in the sunfish family Centrarchidae. Copeia 1932:1-108.
Breder, C. M. and D. E. Rosen. 1966. Modes of reproduction in fishes. Amer. Mus. Nat. Hist. Press, Garden City. 941 p.
Eaton, T. H. 1956. Notes on the olfactory organs in Centrarchidae. Copeia 1956:196-199.
Eddy, S. and J. C. Underhill. 1978. How to know the freshwater fishes. 3rd ed. Wm. C. Brown Co., Dubuque. 215 p.
Farris, D. A. 1959. A change in the early growth rates of four larval marine species. Limnol. Oceanog. 4:29-36.
Hay, O. P. 1880. On a collection of fishes from eastern Mississippi. Proc. U.S. Nat. Mus. 3:488-515.
Helfman, G. S., B. B. Collette and D. E. Facey. 1997. The diversity of fishes. Blackwell Science, Malden, Mass. 528 p.
Humphries, J. M. and G. V. Lauder. 1985. Sunfish phylogeny, outgroups, and percomorph systematics. 65th Ann. Meet. Amer. Soc. Ichthyol. and Herp., p. 78-79 (abstract).
Innes, W. T. 1969. Exotic aquarium fishes. T.F.H. Publications, Inc., Jersey City. 448 p.
Johnson, G. D. 1984. Percoidei: development and relationships. In H. G. Moser et al. (Eds.). Ontogeny and systematics of fishes. Spec. Publ. No. 1, Amer. Soc. Ichthyol. and Herp.: 464-498.
----------. 1993. Percomorph phylogeny: progress and problems. Bull. Mar. Sci. 52:3-28.
---------- and C. Patterson. 1993. Percomorph phylogeny: a survey of acanthomorphs and a new proposal. Bull. Mar. Sci. 52:554-626.
---------- and V. Springer. 1997. Elassoma: another look. 77th Ann. Meet. Amer. Soc. Ichthyol. and Herp., p. 176 (abstract).
Jordan, D. S. 1877. Contributions to North American ichthyology based primarily on the collections of the U.S. Museum. II. Notes on the Cottidae, Etheostomatidae, Percidae, Centrarchidae, Aphredoderidae, Dorysomatidae, and Cyprinidae, with revisions of the genera and descriptions of new or little known species. Bull. U.S. Nat. Mus. 10:1-68.
----------. 1884. List of fishes collected in Lake Jessup and Indian River, Florida, by Mr. R. E. Earll, with descriptions of two new species. Proc. U.S. Nat. Mus. 7:322-324.
---------- and C. H. Gilbert. 1883. Synopsis of the fishes of North America. Bull. U.S. Nat. Mus. 16:1-1068.
Lagler, K. E., J. E. Bardach and R. R. Miller. 1962. Ichthyology. John Wiley and Sons, New York. 545 p.
Mayden, R. L. 1993. Elassoma alabamae, a new species of pygmy sunfish endemic to the Tennessee River drainage of Alabama (Teleostei: Elassomatidae). Bull. Ala. Mus. Nat. Hist. 16:1-14.
Mayr, E. 1969. Principles of systematic zoology. McGraw-Hill Book Co., New York. 428 p.
Mettee, M. F. 1974. A study on the reproductive behavior, embryology, and larval development of the pygmy sunfishes in the genus Elassoma. Ph.D. Dissertation. University of Alabama, Tuscaloosa. 130 p.
---------- and J. J. Pulliam. 1986. Reintroduction of an undescribed species of Elassoma into Pryor Branch, Limestone County, Alabama. Southeastern Fishes Council Proceedings 4:14-15.
Miller, H. C. 1964. The behavior of the pumpkinseed sunfish, Lepomis gibbosus (Linnaeus), with notes on the behavior of other species of Lepomis and the pygmy sunfish Elassoma evergaldei. Behavior. 22: 88-151.
Moore, G. A. and M. E. Sisk. 1963. The spectacle of Elassoma zonatum Jordan. Copeia 1963:346-350.
Nachstedt, J. and H. Tusche. 1954. Breeding aquarium fishes. Aquarium Stock Co., New York. 127 p.
Nelson, J. S. 1994. Fishes of the world. 3rd ed. John Wiley & Sons, New York. 600 p.
Poyser, W. A. 1919. Notes on the breeding habits of the pygmy sunfish. Aquatic Life. 4:65-69.
Roberts, F. L. 1964. A chromosome study of twenty species of Centrarchidae. J. Morph. 115:401-418.
Robins, C. R., R. M. Bailey, C. E. Bond, J. R. Brooker, E. A. Lachner, R. N. Lea, and W. B. Scott. 1991. A list of the common and scientific names of fishes from the United States and Canada. 5th ed. American Fisheries Society Special Publication 20. 183 p.
Rohde, F. C. and R. G. Arndt. 1987. Two new species of pygmy sunfishes (Elassomatidae, Elassoma) from the Carolinas. Proc. Acad. Nat. Sci. Phila. 139:65-85.
Shortt, L. R. 1956. A new pygmy sunfish. The Aquarium. 25:133-135.
Sterba, G. 1961. Freshwater fishes of the world. Pet Library, New York. 879 p.
Walsh, S. J. and B. M. Burr. 1984. Life history of the banded pygmy sunfish, Elassoma
zonatum Jordan (Pisces, Centrarchidae) in western Kentucky. Bull. Ala. Mus. Nat.
Hist. 8:31-52.
© 1998 North American Native Fishes Association. May not be republished without written permission from NANFA.